[Click here for a PDF of this post with nicer formatting (especially if my latex to wordpress script has left FORMULA DOES NOT PARSE errors.)]
Question: Rotation of diatomic molecules ([2] problem 3.6)
In our first look at the ideal gas we considered only the translational energy of the particles. But molecules can rotate, with kinetic energy. The rotation motion is quantized; and the energy levels of a diatomic molecule are of the form

where 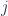 is any positive integer including zero:
is any positive integer including zero:  . The multiplicity of each rotation level is
. The multiplicity of each rotation level is  .
.
a
Find the partition function  for the rotational states of one molecule. Remember that
for the rotational states of one molecule. Remember that  is a sum over all states, not over all levels — this makes a difference.
is a sum over all states, not over all levels — this makes a difference.
b
Evaluate  approximately for
approximately for 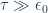 , by converting the sum to an integral.
, by converting the sum to an integral.
c
Do the same for  , by truncating the sum after the second term.
, by truncating the sum after the second term.
d
Give expressions for the energy  and the heat capacity
and the heat capacity 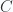 , as functions of
, as functions of  , in both limits. Observe that the rotational contribution to the heat capacity of a diatomic molecule approaches 1 (or, in conventional units,
, in both limits. Observe that the rotational contribution to the heat capacity of a diatomic molecule approaches 1 (or, in conventional units, 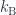 ) when
) when 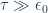 .
.
e
Sketch the behavior of  and
and 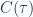 , showing the limiting behaviors for
, showing the limiting behaviors for  and
and  .
.
Answer
a. Partition function 
To understand the reference to multiplicity recall (section 4.13 [1]) that the rotational Hamiltonian was of the form

where the  eigenvectors satisfied
eigenvectors satisfied
\begin{subequations}


\end{subequations}
and  , where
, where  is a positive integer. We see that
is a positive integer. We see that  is of the form
is of the form

and our partition function is

We have no dependence on 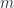 in the sum, and just have to sum terms like fig 1, and are able to sum over
in the sum, and just have to sum terms like fig 1, and are able to sum over 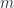 trivially, which is where the multiplicity comes from.
trivially, which is where the multiplicity comes from.

Fig 1: Summation over m
To get a feel for how many terms are significant in these sums, we refer to the plot of fig 2. We plot the partition function itself in, truncation at 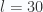 terms in fig 3.
terms in fig 3.

Fig 2: Plotting the partition function summand

Fig 3: Z_R(tau) truncated after 30 terms in log plot
b. Evaluate partition function for large temperatures
If 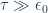 , so that
, so that 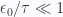 , all our exponentials are close to unity. Employing an integral approximation of the partition function, we can somewhat miraculously integrate this directly
, all our exponentials are close to unity. Employing an integral approximation of the partition function, we can somewhat miraculously integrate this directly

c. Evaluate partition function for small temperatures
When  , so that
, so that  , all our exponentials are increasingly close to zero as
, all our exponentials are increasingly close to zero as  increases. Dropping all the second and higher order terms we have
increases. Dropping all the second and higher order terms we have

d. Energy and heat capacity
In the large 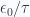 domain (small temperatures) we have
domain (small temperatures) we have

The specific heat in this domain is

For the small 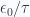 (large temperatures) case we have
(large temperatures) case we have
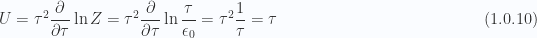
The heat capacity in this large temperature region is

which is unity as described in the problem.
e. Sketch
The energy and heat capacities are roughly sketched in fig 4.

Fig 4: Energy and heat capacity
It’s somewhat odd seeming that we have a zero point energy at zero temperature. Plotting the energy (truncating the sums to 30 terms) in fig 5, we don’t see such a zero point energy.

Fig 5: Exact plot of the energy for a range of temperatures (30 terms of the sums retained)
That plotted energy is as follows, computed without first dropping any terms of the partition function

To avoid the zero point energy, we have to use this and not the truncated partition function to do the integral approximation. Doing that calculation (which isn’t as convenient, so I cheated and used Mathematica). We obtain

This approximation, which has taken the sums to infinity, is plotted in fig 6.

Fig 6: Low temperature approximation of the energy
From eq. 1.0.12, we can take one more derivative to calculate the exact specific heat
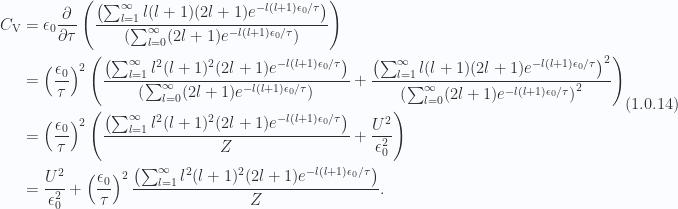
This is plotted to 30 terms in fig 7.

Fig 7: Specific heat to 30 terms
References
[1] BR Desai. Quantum mechanics with basic field theory. Cambridge University Press, 2009.
[2] C. Kittel and H. Kroemer. Thermal physics. WH Freeman, 1980.






